Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 (cr) at 318 K in the presence of iron
(cr) at 318 K in the presence of ironYoshida, Yasushi*; Kitamura, Akira; Shibutani, Sanae*
Journal of Nuclear Science and Technology, 60(8), p.900 - 910, 2023/08
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)Kitamura, Akira
JAEA-Data/Code 2020-020, 164 Pages, 2021/03
Part of JAEA's Thermodynamic Database (JAEA-TDB) for solubility and speciation of radionuclides (JAEA-TDB-RN) for performance assessment of geological disposal of high-level radioactive and TRU wastes has been updated with subsuming the database for geochemical calculations (JAEA-TDB-GC). This report has focused to update JAEA-TDB-RN after selecting change in standard Gibbs free energy of formation (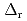
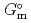 ), change in standard enthalpy change of formation (
), change in standard enthalpy change of formation (
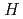

 ), standard molar entropy (
), standard molar entropy (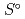
 ) and, heat capacity (
) and, heat capacity (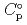 ), change in standard Gibbs free energy of reaction (
), change in standard Gibbs free energy of reaction (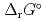
 ), change in standard enthalpy change of reaction (
), change in standard enthalpy change of reaction (
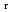
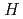

 ) and standard entropy change of reaction (
) and standard entropy change of reaction (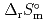 ) as well as logarithm of equilibrium constant (log
) as well as logarithm of equilibrium constant (log
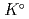 ) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
) at standard state. The extent of selection of these thermodynamic data enables to evaluate solubility and speciation of radionuclides at temperatures other than 298.15 K. Furthermore, the latest thermodynamic data for iron which have been critically reviewed, selected and compiled by the Nuclear Energy Agency within Organisation for Economic Co-operation and Development (OECD/NEA) have been accepted. Most of previously selected log
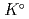 have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
have been refined to confirm internal consistency with JAEA-TDB-GC. Text files of the updated JAEA-TDB have been provided for geochemical calculation programs of PHREEQC and Geochemist's Workbench.
Kitamura, Akira; Yoshida, Yasushi*; Goto, Takahiro*; Shibutani, Sanae*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 27(2), p.58 - 71, 2020/12
Evaluation and estimation of solubility values are required for a performance assessment of geological disposal of high-level radioactive and TRU wastes. Selection of solubility-limiting solid phases (SSPs) that control the solubility of radionuclides is necessary for the evaluation and estimation of solubility values. The authors have developed a methodology for selection of the SSP through a calculation of saturation indices (SIs) using thermodynamic database to show a transparent procedure for the selection. Literature survey should be performed to confirm decision of the SSP from candidate SSPs which generally have larger SIs from realistic point of view for precipitation and solubility control. The authors have selected the SSPs for the elements of interest for the latest Japanese performance assessment in bentonite and cement porewaters after grouping various water compositions.
Yamaguchi, Tetsuji; Ohira, Saki; Hemmi, Ko; Barr, L.; Shimada, Asako; Maeda, Toshikatsu; Iida, Yoshihisa
Radiochimica Acta, 108(11), p.873 - 877, 2020/11
Times Cited Count:7 Percentile:66.68(Chemistry, Inorganic & Nuclear)Kitamura, Akira
Nihon Genshiryoku Gakkai-Shi ATOMO , 62(1), p.23 - 28, 2020/01
, 62(1), p.23 - 28, 2020/01
Thermodynamic databases (TDBs) for performance assessment of geological disposal of high-level waste and TRU waste have been developed to predict solubility and speciation of radionuclides in groundwater in some countries including Japan. The present manuscript briefly describes current status of development of the TDB organized by the Nuclear Energy Agency within the Organisation of Economic Co-operation and Development (OECD/NEA) and the TDBs in some countries including Japan.
 -isosaccharinic acid
-isosaccharinic acidKobayashi, Taishi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Chemical Thermodynamics, 138, p.151 - 158, 2019/11
Times Cited Count:3 Percentile:14.74(Thermodynamics)The effect of  -isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH
-isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH ) range of 6
) range of 6 13 and at total ISA concentration ([ISA]
13 and at total ISA concentration ([ISA]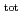 ) = 10
) = 10
 10
10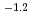 mol/dm
mol/dm . The dependence of U(IV) solubility on pH
. The dependence of U(IV) solubility on pH and [ISA]
and [ISA]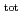 suggested the existence of U(OH)
suggested the existence of U(OH) (ISA)
(ISA)
 as a dominant species within the investigated pH
as a dominant species within the investigated pH range of 6
range of 6 12. For the U(VI)-ISA system, UO
12. For the U(VI)-ISA system, UO (OH)
(OH) (ISA)
(ISA)
 was suggested as a dominant species at pH
was suggested as a dominant species at pH 7
7 13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
Hamamoto, Takafumi*; Ishida, Keisuke*; Shibutani, Sanae*; Fujisaki, Kiyoshi*; Tachi, Yukio; Ishiguro, Katsuhiko*; McKinley, I. G.*
Proceedings of 2019 International High-Level Radioactive Waste Management Conference (IHLRWM 2019) (USB Flash Drive), p.77 - 82, 2019/04
Rai, D.*; Yui, Mikazu; Kitamura, Akira
Progress in Nuclear Science and Technology (Internet), 5, p.19 - 26, 2018/11
The objectives of this presentation are (1) to describe the solubility method, (2) to list desirable criteria of the solubility method so that the reader can recognize which studies have been done in a way that yields quality information, (3) to present an example of how to use the evaluation criteria, and (4) to provide a few examples of future research needs where the solubility method is ideally suited and the other methods are unsuitable for these investigations.
 (am) solubility at 25
(am) solubility at 25 C in the Ca
C in the Ca -Na
-Na -H
-H -Cl
-Cl -OH
-OH -H
-H O system; A Critical review
O system; A Critical reviewRai, D.*; Kitamura, Akira; Altmaier, M.*; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Journal of Solution Chemistry, 47(5), p.855 - 891, 2018/05
Times Cited Count:8 Percentile:8.45(Chemistry, Physical)We have critically reviewed experimental data for Zr hydrolysis constant values for formation of several mononuclear and polynuclear species and a solubility product value for ZrO (am). We have determined new/revised values for the formation constants of Zr(OH)
(am). We have determined new/revised values for the formation constants of Zr(OH)
 , Zr(OH)
, Zr(OH) (aq), Zr(OH)
(aq), Zr(OH)
 , Zr(OH)
, Zr(OH)
 and Ca
and Ca Zr(OH)
Zr(OH)
 , and the solubility product for ZrO
, and the solubility product for ZrO (am) after the critical review.
(am) after the critical review.
 (am) in the aqueous K
(am) in the aqueous K - HCO
- HCO
 - CO
- CO
 - OH
- OH - H
- H O system
O systemRai, D.*; Kitamura, Akira; Rosso, K.*
Radiochimica Acta, 105(8), p.637 - 647, 2017/08
Times Cited Count:2 Percentile:19.65(Chemistry, Inorganic & Nuclear)Solubility of HfO (am) was determined as a function of KHCO
(am) was determined as a function of KHCO concentrations ranging from 0.001 mol.kg
concentrations ranging from 0.001 mol.kg to 0.1 mol.kg
to 0.1 mol.kg . The solubility of HfO
. The solubility of HfO (am) increased dramatically with the increase in KHCO
(am) increased dramatically with the increase in KHCO concentrations, indicating that Hf(IV) makes strong complexes with carbonate. Thermodynamic equilibrium constants for the formation of Hf-carbonate complexes were determined using both the Pitzer and SIT models. The dramatic increase in Hf concentrations with the increase in KHCO
concentrations, indicating that Hf(IV) makes strong complexes with carbonate. Thermodynamic equilibrium constants for the formation of Hf-carbonate complexes were determined using both the Pitzer and SIT models. The dramatic increase in Hf concentrations with the increase in KHCO concentrations can best be described by the formation of Hf(OH-)
concentrations can best be described by the formation of Hf(OH-) (CO
(CO )
)
 and Hf(CO
and Hf(CO )
)
 . The log
. The log K
K values for the reactions [Hf
values for the reactions [Hf + 2 CO
+ 2 CO
 +2 OH
+2 OH
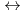 Hf(OH)
Hf(OH) (CO
(CO )
)
 ] and [Hf
] and [Hf + 5 CO
+ 5 CO

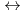 Hf(CO
Hf(CO )
)
 ], based on the SIT model, were determined to be 44.53
], based on the SIT model, were determined to be 44.53  0.46 and 41.53
0.46 and 41.53  0.46, respectively.
0.46, respectively.
Kobayashi, Taishi*; Teshima, Takeshi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Nuclear Science and Technology, 54(2), p.233 - 241, 2017/02
Times Cited Count:6 Percentile:51.46(Nuclear Science & Technology)Zr solubility in the presence of gluconic acid (GLU) and isosaccharinic acid (ISA) was investigated as a function of hydrogen ion concentration (pH ) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH
) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH and GLU concentration suggested the existence of Zr(OH)
and GLU concentration suggested the existence of Zr(OH) (GLU)
(GLU)
 in the neutral pH region and Zr(OH)
in the neutral pH region and Zr(OH) (GLU)(GLU
(GLU)(GLU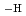 )
) in the alkaline pH region above pH
in the alkaline pH region above pH 10 as the dominant species in the presence of 10
10 as the dominant species in the presence of 10 - 10
- 10 mol/dm
mol/dm (M) GLU. In the presence of ISA, the dominant species Zr(OH)
(M) GLU. In the presence of ISA, the dominant species Zr(OH) (ISA)
(ISA)
 and Zr(OH)
and Zr(OH) (ISA)(ISA
(ISA)(ISA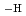 )
) were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH)
were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH) (am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
(am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
 (cr)
(cr)Rai, D.*; Kitamura, Akira; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Radiochimica Acta, 104(8), p.583 - 592, 2016/08
Times Cited Count:5 Percentile:43.41(Chemistry, Inorganic & Nuclear)Solubility studies were conducted with HfO (cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400
(cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400  C, and (4) heating amorphous HfO
C, and (4) heating amorphous HfO (am) suspensions to 90
(am) suspensions to 90  C to ascertain whether the HfO
C to ascertain whether the HfO (am) converts to HfO
(am) converts to HfO (cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO
(cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO (cr) contains a small fraction of less crystalline, but not amorphous, material [HfO
(cr) contains a small fraction of less crystalline, but not amorphous, material [HfO (lcr)] and this, rather than the HfO
(lcr)] and this, rather than the HfO (cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log
(cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log of the solubility product of HfO
of the solubility product of HfO (cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
(cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
 doped with neptunium
doped with neptuniumSato, Tsuyoshi*; Yamashita, Toshiyuki; Matsui, Tsuneo*
Journal of Nuclear Materials, 344(1-3), p.67 - 72, 2005/09
Times Cited Count:3 Percentile:24.22(Materials Science, Multidisciplinary)Phase relationships between NpO and CaTiO
and CaTiO or Ca(Ti, Al)O
or Ca(Ti, Al)O were investigated by X-ray diffraction (XRD) analysis, using specimens prepared at 1773 K in Ar-8%H
were investigated by X-ray diffraction (XRD) analysis, using specimens prepared at 1773 K in Ar-8%H . Single phase solid solutions were formed 0-7.5 mol%Np and 1-10 mol%Np for CaTiO
. Single phase solid solutions were formed 0-7.5 mol%Np and 1-10 mol%Np for CaTiO and Ca(Ti, Al)O
and Ca(Ti, Al)O , respectively. By substituting Al for Ti in CaTiO
, respectively. By substituting Al for Ti in CaTiO , Np solubility in Ca(Ti, Al)O
, Np solubility in Ca(Ti, Al)O increased. Solubility of Np was compared with those of U and Pu, and was discussed with oxidation states and ionic radii of dopants. Thermal expansions of (Ca,Np)TiO
increased. Solubility of Np was compared with those of U and Pu, and was discussed with oxidation states and ionic radii of dopants. Thermal expansions of (Ca,Np)TiO were measured from room temperature to 1273 K in Ar-8%H
were measured from room temperature to 1273 K in Ar-8%H using high-temperature XRD technique. These specimens showed nearly the same value of volumetric thermal expansion coefficients, suggesting that the incorporation of tetravalent Np into CaTiO
using high-temperature XRD technique. These specimens showed nearly the same value of volumetric thermal expansion coefficients, suggesting that the incorporation of tetravalent Np into CaTiO had practically no effect on stabilization of the crystal lattice. This finding was in a marked contrast to that of Pu doped CaTiO
had practically no effect on stabilization of the crystal lattice. This finding was in a marked contrast to that of Pu doped CaTiO , where pronounced stabilization in the crystal was observed by incorporating Pu into CaTiO
, where pronounced stabilization in the crystal was observed by incorporating Pu into CaTiO .
.
Iida, Yoshihisa; Taki, Hiroshi; Yamaguchi, Tetsuji; Tanaka, Tadao; Negishi, Kumi; Nakayama, Shinichi
JAERI-Conf 2005-007, p.230 - 235, 2005/08
A variation in data should be quantitatively incorporated in a probabilistic safety assessment of radioactive waste disposal. We focus our experimental efforts on parameters that induce major uncertainties in the radionuclide migration analysis and that have not been quantitatively assessed. Possible sources of uncertainty includes increase in ionic strength of groundwater caused by intrusion of seawater and/or dissolution of sodium nitrate in TRU waste, highly alkaline conditions originating from cementitious materials, and a variation of porewater composition accompanied with corrosion of. This study is a summary of current status of our investigations for solubility and diffusion.
Nakayama, Shinichi; Iida, Yoshihisa; Nagano, Tetsushi; Akimoto, Toshiyuki
Journal of Nuclear Science and Technology, 40(4), p.227 - 237, 2003/04
Times Cited Count:12 Percentile:62.23(Nuclear Science & Technology)Leaching behavior of synthetic bituminized waste form was studied to inquire data for performance assessment of the geologic disposal. Laboratory-scale leaching tests were performed. The bituminized samples were contacted with an alkaline solution representing cement-contacting groundwater, with a saline solution simulating seawater for a possible repository construction at the coastal area, and with deionized water as reference. The release of soluble components, Na and Cs, was enhanced by the swelling, and considered to be diffusion controlled in the swelled layers of the specimens. The release of insoluble components such as Ba and Np was solubility-limited in addition to control by the progression of leaching. Neptunium, a redox-sensitive element, showed a distinct difference in release reflecting the difference between the anoxic and atmospheric conditions. The concentrations of Pu were below the detection limit (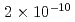 mol/L) under all of the leaching conditions in this study.
mol/L) under all of the leaching conditions in this study.
Ogawa, Hiroaki*; Kiuchi, Kiyoshi
JAERI-Research 2002-037, 48 Pages, 2002/12
The difference in hydrogen permeation among candidate cladding materials such as 25Cr-35Ni stainless steel, Nb liner and reference materials such as 18Cr-8Ni SS, and Zr of Zircaloy base metal were evaluated by low energy plasma permeation simulated to hydrogen excited by heavy neutron irradiation. RF excitation source was arranged for the experimental apparatus in cooperating with temperature and bias control. Comparing with the thermodynamic gas driven permeation (GDP) in the same hydrogen pressure, the hydrogen permeation rate by the plasma driven permeation (PDP) was markedly accelerated at low to medium temperature range. The temperature dependency showed a knick at around 530K due to hydrogen-defect interactions. Comparing with Zr, Nb showed the high hydrogen solubility without the degradation by hydrate formation that is required to a getter material. The difference in PDP among candidates was analyzed with a new dissolution model for hydrogen.
Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Ogawa, Toru
Journal of Alloys and Compounds, 327(1-2), p.235 - 239, 2001/08
Times Cited Count:13 Percentile:60.77(Chemistry, Physical)no abstracts in English
Kimura, Hideo; Takano, Hideki; Muromura, Tadasumi
Journal of Nuclear Materials, 274(1-2), p.197 - 205, 1999/00
Times Cited Count:7 Percentile:49.7(Materials Science, Multidisciplinary)no abstracts in English
Kimura, Hideo; Matsuzuru, Hideo; Takano, Hideki; Muromura, Tadasumi
JAERI-Research 97-049, 25 Pages, 1997/07
no abstracts in English
Fujino, Takeo*; *; Sato, Nobuaki*; Yamada, Kota*; Fukuda, Kosaku; Serizawa, Hiroyuki; Shiratori, Tetsuo
Journal of Nuclear Materials, 246(2-3), p.150 - 157, 1997/00
Times Cited Count:22 Percentile:83.3(Materials Science, Multidisciplinary)no abstracts in English